

#Statistical calculations for trials trial#
Our recommendations do not solve all problems of interpreting results from randomised clinical trials, but we aim to present a valid, practical, relatively simple, and yet comprehensive assessment tool to be used by trial investigators and clinical research consumers.
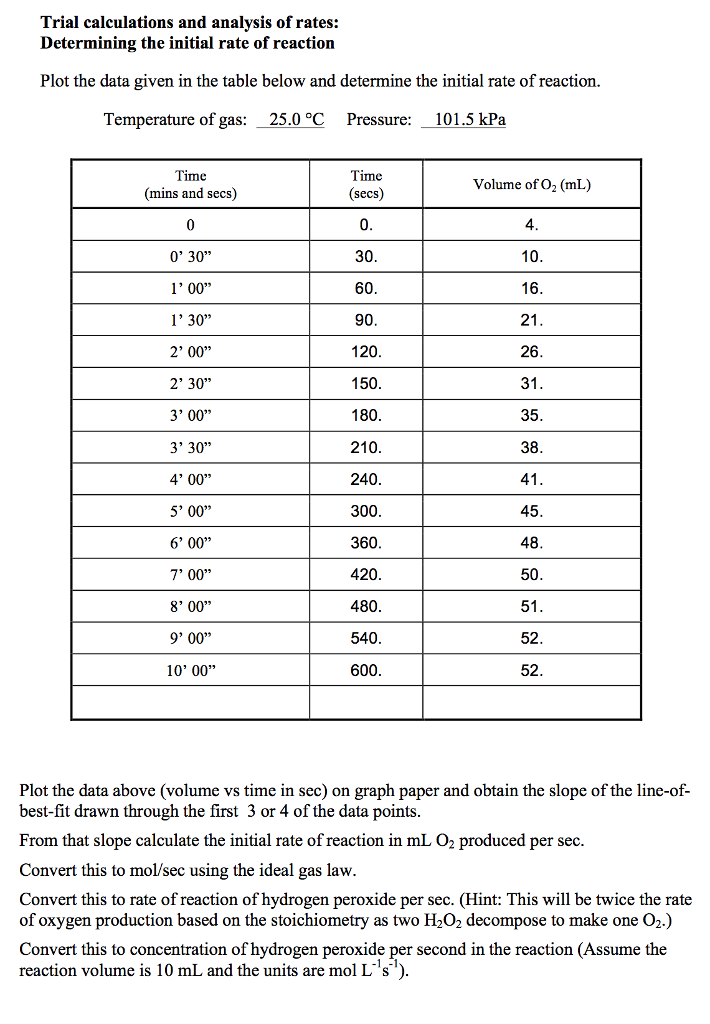
In the following we describe the methodological limitations of focusing too much on confidence intervals and P-values, and suggest a five-step procedure for a more valid assessment of results of intervention effects in randomised clinical superiority trials. P-values are easily calculated but are often misinterpreted and misused. A P-value less than 5% has been the most commonly used threshold for statistical significance in clinical intervention research since Fisher warned against exactly that in 1955. In this approach, a significant difference in effect is declared when a value of a test statistic exceeds a specified threshold showing that it is unlikely that the trial results are produced by zero difference in effect between the compared interventions, i.e., that the null hypothesis is true. Most commonly, the statistical analyses in randomised clinical trials are performed under the frequentist paradigm. The randomised clinical superiority trial remains the mainstay of modern clinical intervention research and is needed for a valid assessment of possible causality between interventions and outcomes. Clinical experience and observational studies cannot and should not be used to validate intervention effects.


 0 kommentar(er)
0 kommentar(er)
